
1998
State of the Art Compounding facility commissioned by Baxter Healthcare in Nashville, TN

2005
AmUSA acquires facility from Baxter

2009
First in industry to receive FDA 510K for irradiated prefilled Saline syringe.
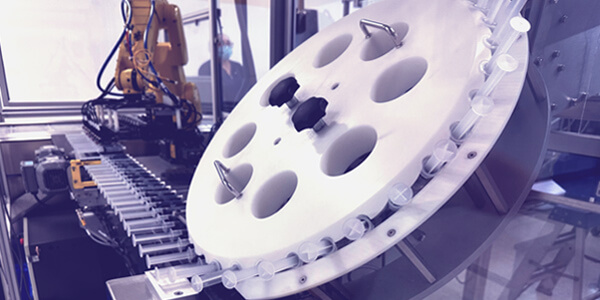
2010
Installation of high-speed filling and packaging lines

2015
Rebranded to Aquabiliti
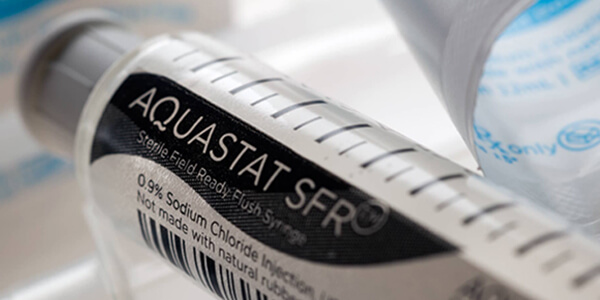
2015
Launch of Sterile Field Ready (SFR) 10ml Saline Flush Syringe

2022
Acquisition of additional plant in PA
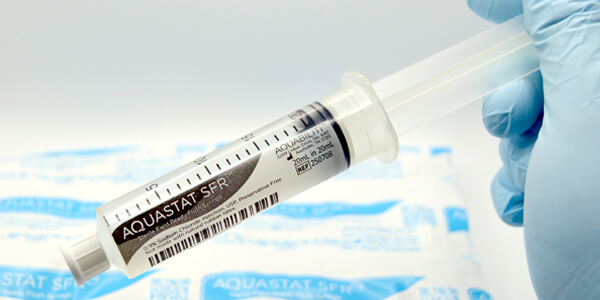
2023
Launch of Sterile Field Ready (SFR) 20ml Saline Flush Syringe

1998
State of the Art Compounding facility commissioned by Baxter Healthcare in Nashville, TN

2005
AmUSA acquires facility from Baxter

2009
First in industry to receive FDA 510K for irradiated prefilled Saline syringe.

2010
Installation of high-speed filling and packaging lines

2015
Rebranded to Aquabiliti

2015
Launch of Sterile Field Ready (SFR) 10ml Saline Flush Syringe

2022
Acquisition of additional plant in PA

2023
Launch of Sterile Field Ready (SFR) 20ml Saline Flush Syringe

1998
State of the Art Compounding facility commissioned by Baxter Healthcare in Nashville, TN

2005
AmUSA acquires facility from Baxter

2009
First in industry to receive FDA 510K for irradiated prefilled Saline syringe.

2010
Installation of high-speed filling and packaging lines

2015
Rebranded to Aquabiliti

2015
Launch of Sterile Field Ready (SFR) 10ml Saline Flush Syringe

2022
Acquisition of additional plant in PA

2023
Launch of Sterile Field Ready (SFR) 20ml Saline Flush Syringe
